We use cookies to improve and analyse your browsing experience on our web. You can accept these cookies, reject them or choose your settings by clicking on the corresponding buttons. Please note that rejecting cookies may affect your browsing experience. For more information you can consult our Cookies policy.
Cookies are an essential part of how our web works. The main goal of cookies is to make your browsing experience more comfortable and efficient and to improve our services and the web itself.
Here you can find all the information about the cookies we use and you can activate and/or deactivate them according to your preferences, except for those cookies that are strictly necessary for the operation of the web. Blocking some cookies may affect your experience on the web and how the site works. For more information you can visit our Cookie Policy.
These Cookies are necessary for the web to function and cannot be disabled on our systems. They are generally only set up in response to actions you may take such as requesting services, setting your privacy preferences, logging in or completing forms. You can set your browser to block or warn you about these cookies, but some parts of the web will not work. Information about Cookies.
These Cookies allow us to count the number of visits and traffic sources so that we can measure and improve the performance of our site. They help us to find out which pages are the most popular and least popular, and to see how visitors move around the web. All information collected by these Cookies is aggregated and therefore anonymous. If you do not allow these Cookies we will not know when you visited our web. Information about Cookies.
These cookies are used to analyse your activity in order to show you personalised advertisements. Information about Cookies.
Change theme

Revision mode

Matter is all around us. We understand that it is anything that has mass and occupies a space, that is, everything that we can find in the Universe, whether visible like a rock or invisible like air.

If we look at everything around us, we observe that matter can be found in three states; solid (like our desk), liquid (like the ink in our pen) and gaseous (like the air we breathe).
Let us look at some experiments that will help us demonstrate the main properties of each state of matter.

Take a stone and put it into a beaker and then into a measuring cylinder. Answer these questions:
a) Does it have mass? Can you weigh it on a set of scales?
When you change the container:
b) Does the mass of the stone change?
c) Is its volume different?
d) Does its shape change?

Put 75 ml of water with some food colouring into a beaker and pour it very carefully into a measuring cylinder without dripping any of it or leaving any water in the beaker. Answer these questions:
a) Does it have mass? Can you weigh it on a set of scales?
When you change the container:
b) Does the mass of the coloured liquid change?
c) Is its volume different?
d) Does its shape change?
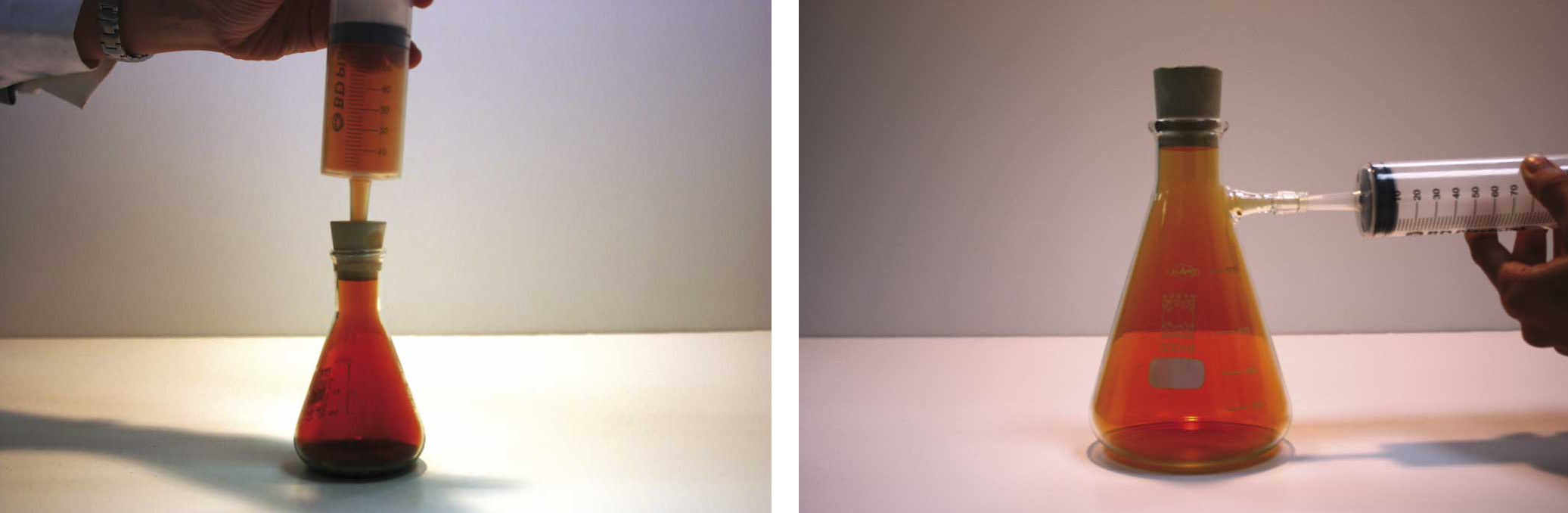
Now look at the next pair of photos. We put a five cent coin into nitric acid (HNO3), which creates nitrogen dioxide (NO2), a brownish gas that can be collected in a syringe. Let's take 50 cm3 of this gas and inject it very carefully into a different container (hermetically sealed with a rubber top). Answer these questions:
a) Does it have mass?
When we change the container:
b) Does the mass of the gas change?
c) Is its volume different?
d) Does its shape change?
The states of matter have properties that are very different from each other and that makes them behave in different ways.
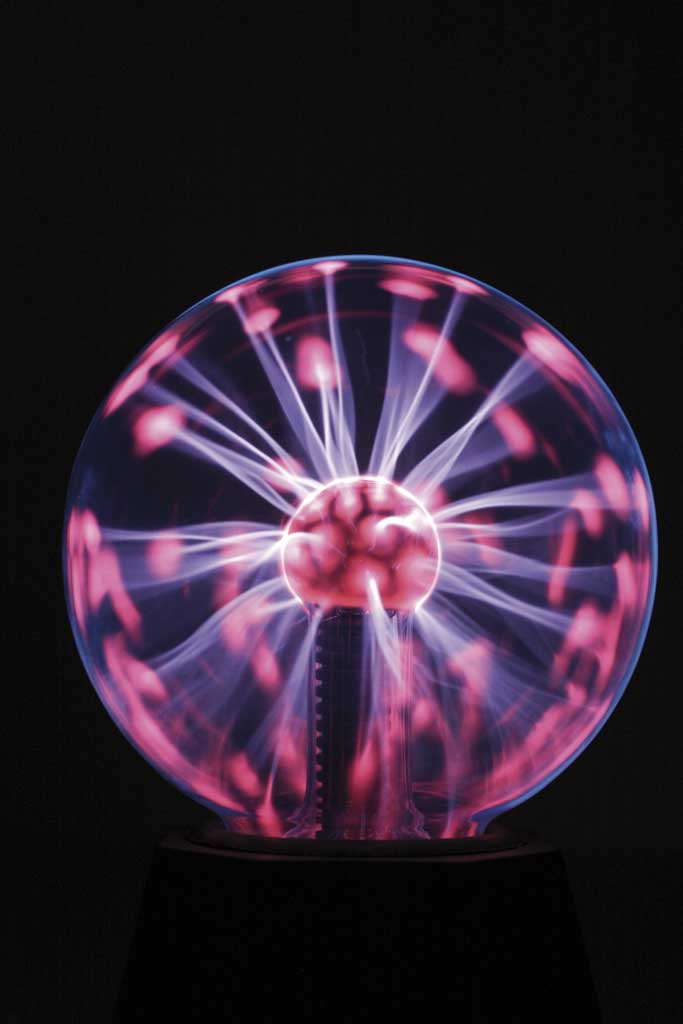
You may have seen a plasma ball or perhaps you have a plasma TV at home. Plasma is considered the fourth state of matter. It occurs when matter is exposed to extremely high temperatures. You may not have heard of it because it's not easy to find on the Earth, but in the Universe almost all matter is in this state. For example, it's the main component of the nucleus of stars.

Solids
The cube doesn't change shape or mass, even if we put it in a different container.
The same is true for its volume; it doesn't change.

Liquids
Water changes shape if we transfer it from one container to another.
However, its mass and volume don't change. So if we have a litre at the beginning, it will always be a litre.

Gases
The air in these balloons, as with all gases, has mass and volume.
Both the volume and the shape will depend on the shape of the container holding it.
This table summarises the most important properties:
| Solids | Liquids | Gases |
|---|---|---|
| Definite mass | Definite mass | Definite mass |
| Invariable shape | Variable shape | Variable shape |
| Constant volume | Constant volume | Variable volume |
| Cannot be compressed | Cannot be compressed | Easy to compress |
| Impenetrable | Penetrable | Penetrable |
As you can see, all liquids and gases change their shape according to the container holding them. However, only gases take up the entire volume of the container they are in (we can observe this if we open a bottle of perfume in one corner of the classroom; after a short period of time, we notice the smell all around the room).
Activity 1
Are there any properties that are repeated in the three states of matter?
Activity 2
Which state do you think has the highest density? Which has the lowest?

