We use cookies to improve and analyse your browsing experience on our web. You can accept these cookies, reject them or choose your settings by clicking on the corresponding buttons. Please note that rejecting cookies may affect your browsing experience. For more information you can consult our Cookies policy.
Cookies are an essential part of how our web works. The main goal of cookies is to make your browsing experience more comfortable and efficient and to improve our services and the web itself.
Here you can find all the information about the cookies we use and you can activate and/or deactivate them according to your preferences, except for those cookies that are strictly necessary for the operation of the web. Blocking some cookies may affect your experience on the web and how the site works. For more information you can visit our Cookie Policy.
These Cookies are necessary for the web to function and cannot be disabled on our systems. They are generally only set up in response to actions you may take such as requesting services, setting your privacy preferences, logging in or completing forms. You can set your browser to block or warn you about these cookies, but some parts of the web will not work. Information about Cookies.
These Cookies allow us to count the number of visits and traffic sources so that we can measure and improve the performance of our site. They help us to find out which pages are the most popular and least popular, and to see how visitors move around the web. All information collected by these Cookies is aggregated and therefore anonymous. If you do not allow these Cookies we will not know when you visited our web. Information about Cookies.
These cookies are used to analyse your activity in order to show you personalised advertisements. Information about Cookies.
Change theme

Revision mode

If you had a mixture of iron and aluminium filings, how would you separate them? We often find that we have homogeneous and heterogeneous mixtures in which we have to separate the components without altering the nature of the pure substances in the process.
The most common methods for separating the components of a mixture are:

You may have thought of using a magnet as the easiest and quickest way to separate the iron from the aluminium and you'd be right. If you don't have a magnet, you could use magnetic fasteners on handbags or the covers for mobile phones as they both possess magnetic properties. The iron is attracted to the magnet and the aluminium isn't.
This method for separating the two components of a heterogeneous mixture is called magnetic separation. It can only be used when one of the components has magnetic properties (like iron) and the others don't.
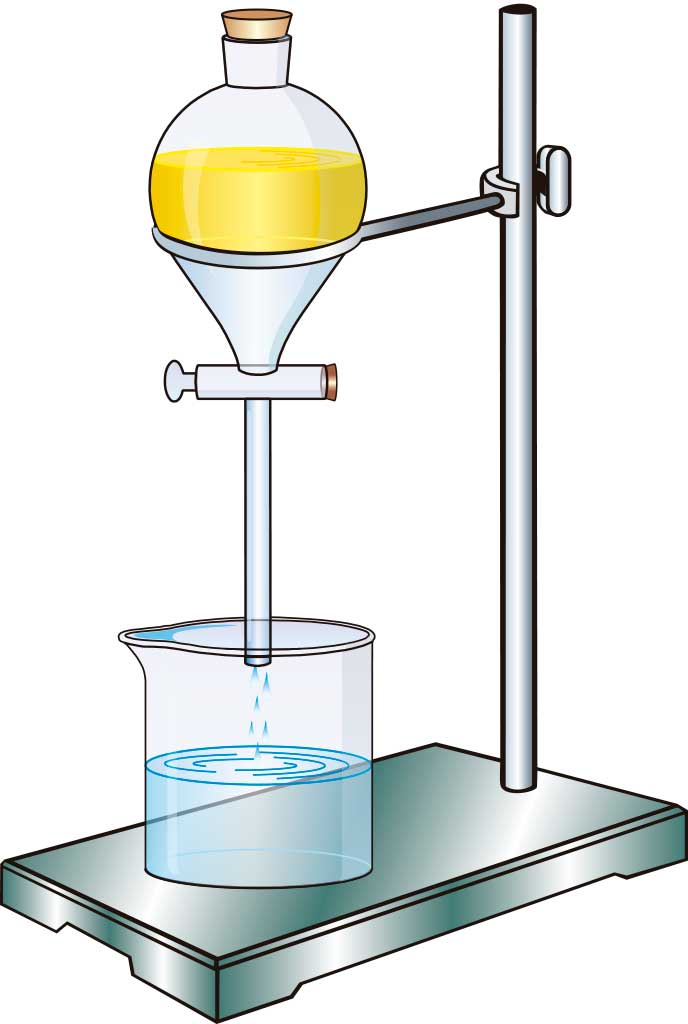
We use decantation for liquids with different densities that don't mix together (immiscible), such as oil and water. For this, you use a decantation funnel:

We use this method to separate a solid from a liquid that hasn't dissolved (insoluble), such as sand and water. To do this, we pass the heterogeneous mixture through a filter with the correct pore size (smaller than the particles that we want to separate). The filter usually goes through a funnel.
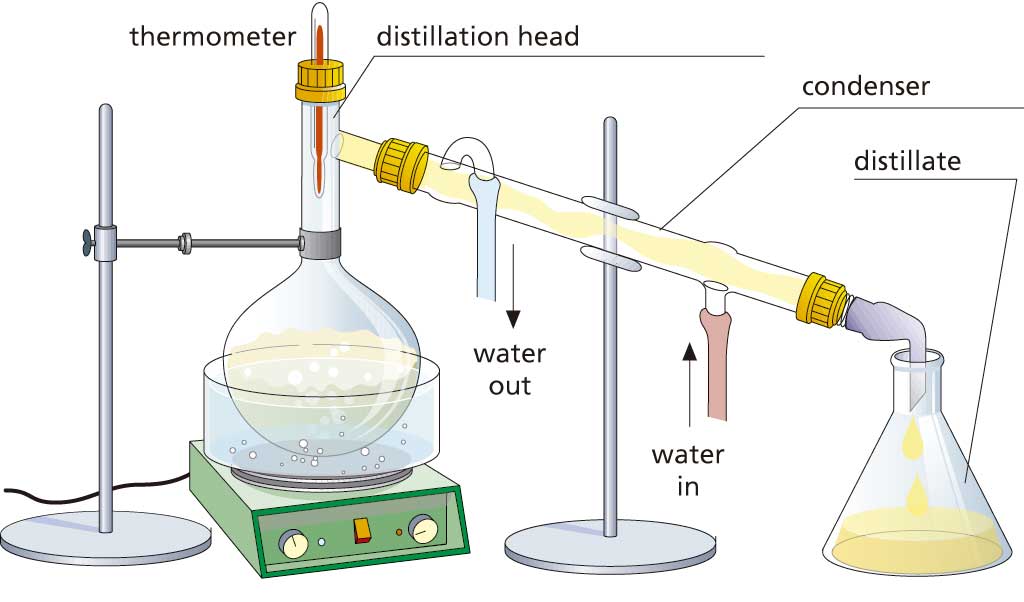
We use distillation to separate soluble liquids with very different boiling points from each other, such as water and alcohol.
Put the mixture into a round-bottom distillation flask and heat it up. When it reaches the lower boiling point of one of the two substances, this substance will turn to vapour and pass through the condenser, where it will cool down and condense. The resulting liquid, called the distillate, is collected in a container (a beaker, for example).
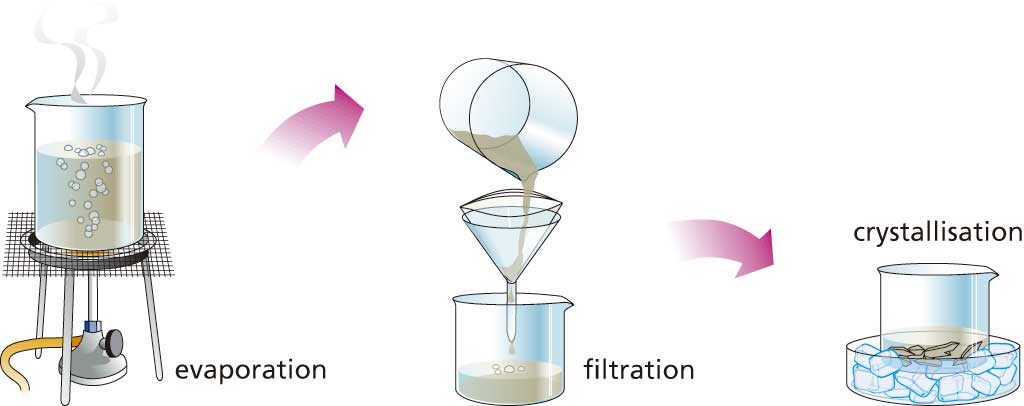
We use this method to separate a solid dissolved in a liquid, such as salt in water. The process starts with evaporating (naturally or forced via heat) the solvent and ends with the depositing of the solid in the form of crystals at the bottom of the container (usually a crystalliser). The slower the evaporation of the solvent, the larger the crystals will be.
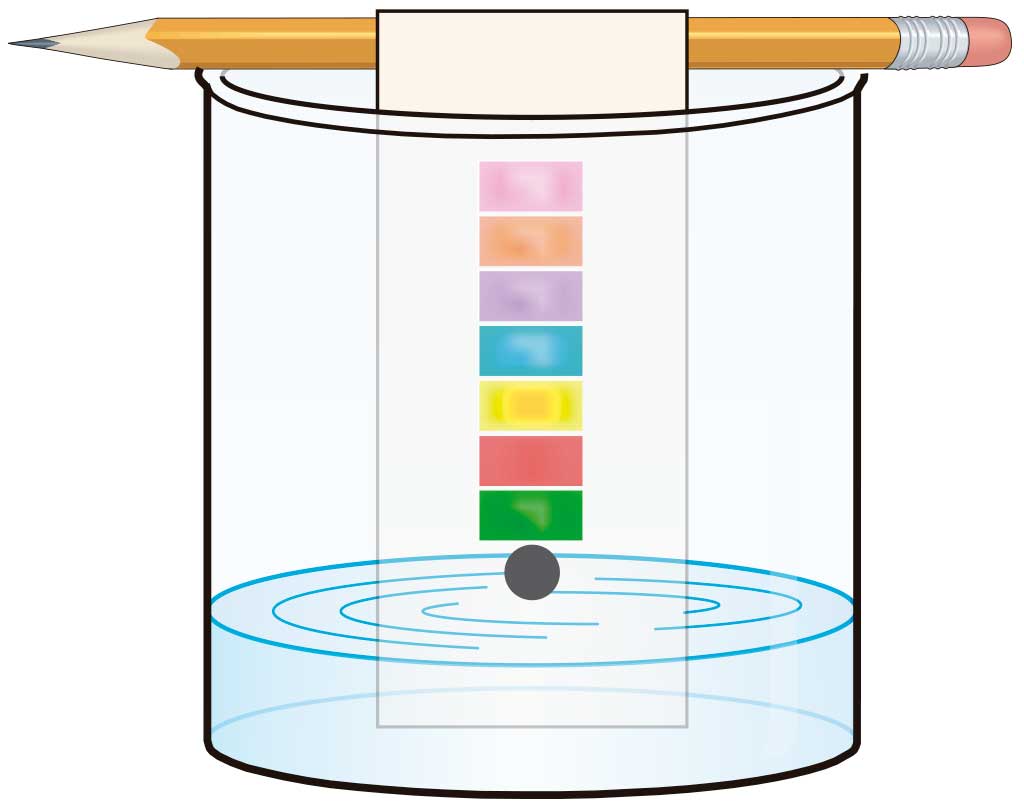
We use this to separate two components in a mixture according to how soluble they are in a particular solvent.
One of the simplest techniques is chromatography in paper, which uses a strip of filter paper.
Put a tiny drop of the mixture onto the strip of filter paper and put the bottom part of the paper into a solvent, such as alcohol. This will move slowly up the filter paper by capillary action, pulling the different components of the mixture with it. Because each component dissolves to a greater or lesser extent in a particular solvent, those that are higher up the strip at the end of the process are more soluble than those at the bottom. This method can be used, for example, to separate photosynthetic pigments (chlorophyll and carotenes) present in spinach and other vegetables.
Activity 28
How would you separate the components in a mixture of oil and vinegar?
Activity 29
How would you separate the components in a mixture of sawdust and water?
Activity 30
Can you think of a way to separate the different colours used to make up the black ink in a marker pen?
Activity 31
How would you separate the components in a mixture of sulphur and water, given that sulphur is insoluble in water? Choose the correct option.

